- Home
- Pharmaceuticals
- Hyaluronic Acid
Hyaluronic Acid
Product Name: Hyaluronic Acid
Synonyms: Hyaluronic Acid powder, Natural Hyaluronic Acid, Sodium hyaluronate, Hyaluronan, HA powder
CAS: 9067-32-7
MF: (C14H22NNaO11)n
Appearance: White powder
Standard: EP7.0
MSDS: MSDS for Sodium Hyaluronate
Read More: http://www.hyaluronicacidsupplier.com
- Description
Description
We Supply
Stanford Chem is a United States wholesale distributor and manufacturer of pure organic hyaluronic acid. Our products are divided by use:
- Medical Grade HA
- Cosmetic Grade HA
- Food Grade HA
- Injection Grade HA
- Cross-linked HA
* Samples are available.
Each product is divided into high, medium, and low molecular weights. See the product table below for details.
Hyaluronic Acid Powder for Sale:
| Main Product | Item No. | Specification |
|
Food Grade Sodium Hyaluronate |
HAF-Micro-SC |
M.W: <5 K Da Ultra Low Molecular Weight |
| HAF-Oligo-SC |
M.W: 5-10 K Da Low Molecular Weight |
|
| HAF-N-SC | M.W: 200-600 K Da | |
| HAF-L-SC | M.W: 100-1,000 K Da(800 KDa) | |
| HAF-M-SC |
M.W: 800-1,600 K Da Middle Molecular Weight |
|
| HAF-H-SC | M.W: >1,800 K Da
High Molecular Weight |
|
| HAF-C-SC | Customized Molecular weight | |
|
Cosmetic Grade Sodium Hyaluronate |
HAC-Micro-SC | M.W: <5 K Da
Ultra Low Molecular Weight |
| HAC-Oligo-SC |
M.W: 5-10 K Da Low Molecular Weight |
|
| HAC-N-SC | M.W: 200-600 K Da | |
| HAC-L-SC | M.W: 100-1,000 K Da(800 KDa) | |
| HAC-M-SC |
M.W: 800-1,600 K Da Middle Molecular Weight |
|
| HAC-H-SC | M.W: >1,800 K Da
High Molecular Weight |
|
| HAC-C-SC | Customized Molecular weight | |
|
Medical Grade Sodium Hyaluronate |
HA-EM2.0-SC | M.W: 800K-1,300K Da,
I.V.: 1.44-2.12 m3/kg |
| HA-EM2.4-SC |
M.W: 1,300K-1,800K Da; I.V: 2.12-2.72 m3/kg |
|
| HA-EM3.0-SC |
M.W:1,800K-2,500K Da; I.V.: 2.72-3.53 m3/kg |
|
| HA-EMC-SC | Customized Molecular weight | |
|
Injection Grade Sodium Hyaluronate |
HA-EP1.8-SC | M.W: 800K-1,300K Da,
I.V.: 1.44-2.12 m3/kg |
| HA-EP2.4-SC |
M.W: 1,300K-1,800K Da; I.V: 2.12-2.72 m3/kg |
|
| HA-EP3.0-SC |
M.W:1,800K-2,500K Da; I.V.: 2.72-3.53 m3/kg |
|
| HA-EPC-SC | Customized Molecular weight |
In addition to these sodium hyaluronate powders, we also offer Cross-linked HA with greater molecular weight, more stable form, better resistance to degradation, and longer lasting moisturizing effect.
Why Choose Us
Our sodium hyaluronate powder is produced by bacterial fermentation technology, ensuring a 100% non-animal hyaluronic acid source. It is vegan-friendly and kosher compliant, meeting the highest purity standard for various applications.
As one of the leading suppliers of hyaluronic acid source, we are involved in offering high-quality sodium hyaluronate raw materials to the USA, Canada, and European buyers. The industries range from food supplements, cosmetics, eye drops, and pharmaceuticals.
Our products have obtained relevant certifications, which shows the safety of the products.
- ISO 9001 (Quality Management System)
- ISO 14001 (Environmental Management System)
- ISO 22000 (Food Safety Management System)
Note: ISO sets standards, and certification bodies such as QAC are responsible for evaluating whether companies meet these standards and issuing corresponding certifications.
5 Uses of Sodium Hyaluronate Powder
Food Grade Hyaluronic Acid / Hyaluronic Acid for Supplement
- Common food: beverage, jelly, dairy products, etc.
- Health food: usually in combination with Collagen, Vitamins, Chondroitin Sulphate, or Glucosamine to make tablets, capsules, or oral liquids these popular forms
- Skin care products: cream, emulsion, essence, lotion, gel, facial mask, etc.
- Beauty products: lipstick, eye shadow, foundation, etc.
- Cleansing products: facial cleanser, body wash, etc.
- Hair products: shampoo, hair conditioner, styling gel, hair restorer, etc.
- For parenteral preparations or oral preparations not including intra-articular and intra-ocular preparations.
- It can be used in eye drops, contact lens solutions, topical preparations for wound or burn healing, medical lubricants, etc.
- Medical Grade Sodium Hyaluronate COA
Injection Grade Hyaluronic Acid
- For parenteral preparations including intra-articular and intra-ocular preparations, such as OVD, intra-articular injections, intradermal injections for aesthetic correction, anti-adhesive products, etc.
- injectable-grade sodium hyaluronate powder COA
Cross-linked Hyaluronic Acid Powder
- Aesthetic Medicine: Used for wrinkle filling and facial contouring with long-lasting effects.
- Orthopedics: Injected to treat arthritis, lubricate joints, and relieve pain.
- Surgical Repair: Reduces scarring and promotes wound healing.
- Ophthalmology: Used as a viscoelastic agent in cataract and vitreous surgeries.
- Tissue Engineering: Serves as a scaffold material to support soft tissue repair.
- Drug Delivery: Acts as a carrier for controlled drug release.
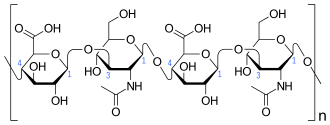
What is Hyaluronic Acid?
Hyaluronic acid is a naturally occurring substance in the human body, primarily found in the fluids of the eyes and joints. Its main functions include retaining moisture and ensuring proper lubrication and hydration of joint tissues. Additionally, it offers various benefits, such as promoting wound healing.
Hyaluronic Acid Specifications
With injectable-grade hyaluronic acid powder as the reference data, it represents the highest quality standard. The table below shows the specifications of product HA-EP2.4-SC.
| Item | Certificate of Analysis Details | Results |
| Characters | White or almost white powder or fibrous aggregate | White powder |
| Identification A(Infrared absorption) | Complies with Ph. Eur. Reference spectrum of Sodium hyaluronate | Complies |
| Identification B(Reaction (a) of sodium) | Positive | Positive |
| Appearance of Solution | Clear (A600nm≤0.01) | 0.000 |
| pH | 5.0 – 8.5 | 6.4 |
| Intrinsic Viscosity | 2.2 – 2.8 m3/kg | 2.40 m3/kg |
| Nucleic Acids | A260nm≤0.5 | 0.006 |
| Protein | ≤ 0.1% | <0.003% |
| Chlorides | ≤ 0.5% | <0.5% |
| Iron | ≤ 30 ppm | 2 ppm |
| Loss on Drying | ≤ 18.0% | 8.0% |
| Microbial Contamination (TAMC) | ≤ 100 cfu/g | <20 cfu/g |
| Bacterial Endotoxins | <0.05 IU/mg | <0.05 IU/mg |
| Residual Solvents (Ethanol) | ≤ 5000 ppm | 99 ppm |
| Assay | 95.0% – 105.0% (dried substance) | 100.4% |
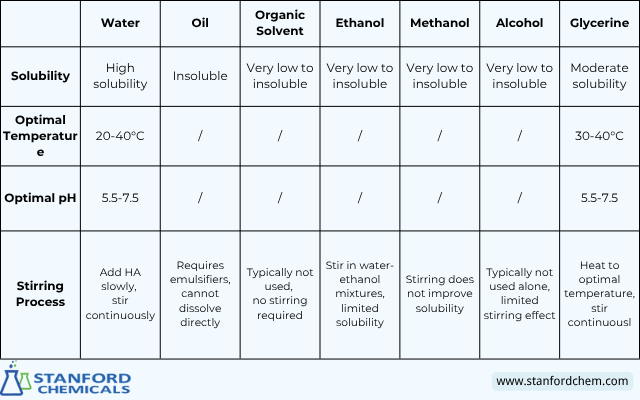
Hyaluronic Acid Solubility
Hyaluronic Acid Benefits
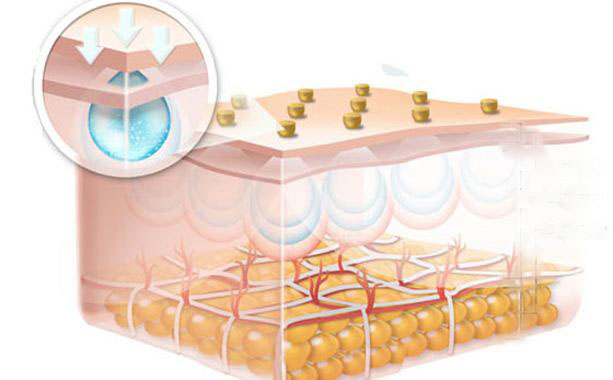
1. Hyaluronic Acid for Skin: Approximately half of the body’s hyaluronic acid is found in the skin, where it binds with water to maintain hydration. Applied topically, it helps reduce wrinkles, redness, and dermatitis. Dermatologists often use hyaluronic acid fillers to keep the skin firm and youthful.
2. Accelerates Wound Healing: Hyaluronic acid naturally occurs in the skin, with its concentration increasing at injury sites to aid repair. Sodium hyaluronate also has antibacterial properties, reducing the risk of infection when applied directly to wounds.
3. Relieves Joint Pain: Hyaluronic acid helps maintain proper lubrication between bones, reducing joint pain and improving mobility.
4. Eases Acid Reflux Symptoms: Studies have shown that a mixture of hyaluronic acid and chondroitin sulfate can promote the healing of acid-damaged throat tissues.
5. For Eye: Alleviates Dry Eyes and Discomfort. Eye drops containing 0.2-0.4% hyaluronic acid have been proven to reduce symptoms of dry eyes and improve overall eye health.
6. Supports Bone Strength
7. Prevents Bladder Pain
People Also Ask
1. What is the difference between medical-grade and cosmetic-grade hyaluronic acid?
Medical grade is of higher purity and safety standards and is used in medical applications, while cosmetic grade is more focused on function and end-user perception and is used for routine skin care.
2. What is the difference between food-grade hyaluronic acid and medical-grade oral products?
Food grade is applied to normal foods and falls under loose regulations, while medical grade oral products need more rigorous drug standards, including drug purity and bioavailability testing.
3. Why do hyaluronic acids with different molecular weights have such different effects on skin?
Molecular weight directly affects skin penetration rate and the mechanism of action.
- High molecular weight mainly forms a moisturizing film on the skin surface;
- Medium molecular weight can penetrate the stratum corneum;
- Low molecular weight can reach the dermis to stimulate collagen regeneration;
- Oligomeric HA (<10kDa) even has cell signaling regulation functions.
4. What is the essential difference between microbial fermentation and animal extraction methods?
The fermentation method uses genetically engineered strains (such as Streptococcus) cultured in a sterile environment, with high product purity and no animal-derived risks; the traditional extraction method extracts from tissues such as rooster combs and is prone to residual heterologous proteins. It has now been phased out by the mainstream market.
Not sure what to ask? Check these out:
The Comprehensive Guide to Hyaluronic Acid
What is Hyaluronic Acid Powder? Benefits and Usage
How is Hyaluronic Acid Powder Made
Solubility of Hyaluronic Acid in Different Solvents and Its Influencing Factors
Top 10 Benefits of Hyaluronic Acid
What Are the Top Benefits of Micro Hyaluronic Acid for Your Skin?
Submit your review | |
1 2 3 4 5 | |
Submit Cancel | |
It really helps my skin.