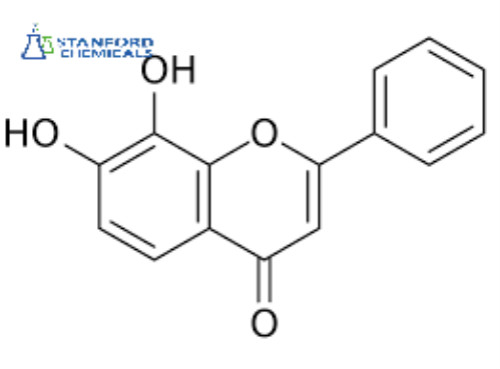
7,8-Dihydroxyflavone Description
7,8-Dihydroxyflavone (7,8-DHF) is a naturally occurring flavone found in Godmania aesculifolia, Tridax procumbens, and primula tree leaves. It has been found to act as a potent and selective small-molecule agonist of the tropomyosin receptor kinase B (TrkB) (Kd ≈ 320 nM), the main signaling receptor of the neurotrophin brain-derived neurotrophic factor (BDNF). 7,8-DHF is both orally bioavailable and able to penetrate the blood-brain barrier.
7,8-Dihydroxyflavone Specifications
| Product Name |
7,8-Dihydroxyflavone |
| CAS Registry Number |
38183-03-8 |
| Molecular Formula |
C15H10O4 |
| Molecular Weight |
254.24 |
| Assay |
≥98% |
| Solubility |
DMSO: 24 mg/mL |
7,8-Dihydroxyflavone Applications
7,8-Dihydroxyflavone has demonstrated therapeutic efficacy in animal models of a variety of central nervous system disorders, including depression, Alzheimer's disease, cognitive deficits in schizophrenia, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis, traumatic brain injury, cerebral ischemia, fragile X syndrome, and Rett syndrome. 7,8-DHF also shows efficacy in animal models of age-associated cognitive impairment and enhances memory consolidation and emotional learning in healthy rodents.
In addition, 7,8-Dihydroxyflavone possesses powerful antioxidant activity independent of its actions on the TrkB receptor and protects against glutamate-induced excitotoxicity, 6-hydroxydopamine-induced dopaminergic neurotoxicity, and oxidative stress-induced genotoxicity. It was also found to block methamphetamine-induced dopaminergic neurotoxicity, an effect which, in contrast to the preceding, was found to be TrkB-dependent.[1]
7,8-Dihydroxyflavone has proven to be a viable therapy option for TBI and multiple degenerative neurological disorders. Through its activation of the TrkB receptor and downstream signaling pathway, it promotes survival and dendritic integrity of neurons, reduces injury-induced tissue damage, and ameliorates motor and cognitive functional impairments. Its ability to cross the BBB and broad therapeutic potential including possible antiinflammation effect in the CNS makes it a valuable compound deserving further examination for its application for TBI and other neurological diseases in the clinic.[2]
The BDNF mimetic compound 7,8-dihydroxyflavone (7,8-DHF), a potent small molecular TrkB agonist, displays prominent therapeutic efficacy against Alzheimer’s disease (AD). However, 7,8-DHF has only modest oral bioavailability and a moderate pharmacokinetic (PK) profile. To alleviate these preclinical obstacles, we used a prodrug strategy for elevating 7,8-DHF oral bioavailability and brain exposure and found that the optimal prodrug R13 has favorable properties and dose-dependently reverses the cognitive defects in an AD mouse model. We synthesized a large number of 7,8-DHF derivatives via ester or carbamate group modification on the catechol ring in the parent compound. Using in vitro absorption, distribution, metabolism, and excretion assays, combined with in vivo PK studies, we identified a prodrug, R13, that prominently up-regulates 7,8-DHF PK profiles. Chronic oral administration of R13 activated TrkB signaling and prevented Aβ deposition in 5XFAD AD mice, inhibiting the pathological cleavage of APP and Tau by AEP. Moreover, R13 inhibited the loss of hippocampal synapses and ameliorated memory deficits in a dose-dependent manner. These results suggest that the prodrug R13 is an optimal therapeutic agent for treating AD.[3]
Reference
J.Romeika, M.Wurzelmann, D.Sun: Chapter 14 - TrkB Receptor Agonist 7,8-Dihydroxyflavone and Its Therapeutic Potential for Traumatic Brain Injury. New Therapeutics for Traumatic Brain Injury-Prevention of Secondary Brain Damage and Enhancement of Repair and Regeneration 2017, Pages 225-234
Chun Chen, Zhihao Wang, Zhentao Zhang, Xia Liu, Seong Su Kang, Ying Zhang, and Keqiang Ye: The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. https://doi.org/10.1073/pnas.1718683115
[1] https://en.wikipedia.org/wiki/7,8-Dihydroxyflavone#cite_note-pmid29295929-10
[2] J.Romeika, M.Wurzelmann, D.Sun: Chapter 14 - TrkB Receptor Agonist 7,8-Dihydroxyflavone and Its Therapeutic Potential for Traumatic Brain Injury. New Therapeutics for Traumatic Brain Injury-Prevention of Secondary Brain Damage and Enhancement of Repair and Regeneration 2017, Pages 225-234
[3] Chun Chen, Zhihao Wang, Zhentao Zhang, Xia Liu, Seong Su Kang, Ying Zhang, and Keqiang Ye: The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. https://doi.org/10.1073/pnas.1718683115