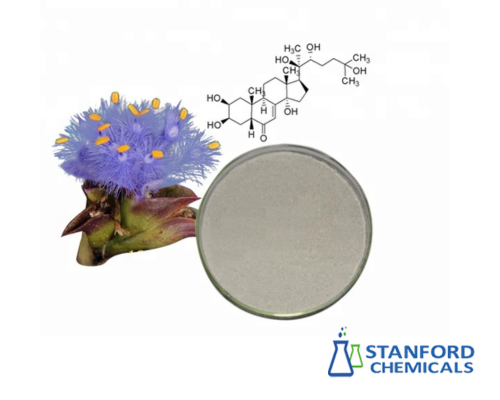
Artemisinic Acid Powder (CAS No. 80286-58-4) Description
Artemisinic Acid Powder (CAS No. 80286-58-4) is a white crystalline sesquiterpene compound naturally abundant in Artemisia annua (sweet wormwood). Serving as the key biosynthetic precursor to artemisinin, it features a characteristic peroxide-free structure with a carboxylic acid functional group. Industrially valued for its role in semi-synthetic artemisinin production, it also exhibits intrinsic anti-inflammatory and antimicrobial bioactivity.
Physicochemical Properties
- Appearance: White to off-white crystalline solid with a subtle herbal aroma
- Melting Point: 142–145°C (decomposition >200°C)
- Solubility:
- Artemisinic Acid Powder demonstrates limited solubility in water but dissolves readily in polar organic solvents, including methanol, ethanol, acetone, and dimethyl sulfoxide, while remaining insoluble in non-polar solvents like hexane or petroleum ether.
- Molecular Formula: C₁₅H₂₂O₂ (sesquiterpene lactone precursor)
-
- Structural Features:
- Decalin core with a carboxylic acid (–COOH) group
- Conjugated double bond (susceptible to oxidation and isomerization)
- Crystal Structure: Orthorhombic, forms hydrogen-bonded dimers
Stability & Handling
- Light Sensitivity: Degrades under prolonged UV/visible light exposure
- Oxidation Risk: Exocyclic methylene group prone to air/moisture-induced yellowing
- Storage Recommendations:
- Temperature: 2–8°C (long-term), ≤25°C (short-term)
- Packaging: Amber glass vials under inert gas (N₂/Ar)
- Particle Characteristics:
- Needle-to-prismatic crystals
- Bulk density: ~0.5 g/cm³
Spectroscopic Data
| Technique |
Key Identifiers |
| UV-Vis |
λ<sub>max</sub> 210 nm, 260 nm |
| FTIR |
3200 cm⁻¹ (O–H), 1700 cm⁻¹ (C=O), 1640 cm⁻¹ (C=C) |
Artemisinic Acid Powder (CAS No. 80286-58-4) Specifications
Properties
| Property |
Value |
| Material |
Artemisinic Acid |
| CAS No. |
80286-58-4 |
| Molecular Formula |
C15H22O2 |
| Purity |
≥98% (HPLC) |
| Appearance |
Colorless Crystal |
| Melting Point |
129-131°C |
| Boiling Point |
373.6°C at 760 mmHg |
*The above product information is based on theoretical data. For specific requirements and detailed inquiries, please contact us.
Applications of Artemisinic Acid Powder
1. Pharmaceutical Manufacturing
- Primary Use: Key intermediate in semi-synthetic artemisinin production for antimalarial drugs (e.g., artesunate, dihydroartemisinin)
- Advantage: Cost-effective alternative to botanical extraction
2. Cosmetics & Skincare
- Anti-Aging: Neutralizes free radicals, reduces UV-induced collagen degradation
- Formulations: Serums, creams with 0.5–2% concentration
3. Agricultural Solutions
- Biopesticide: Controls fungal pathogens (e.g., Botrytis cinerea) and insect larvae
- Crop Benefits: Residue-free, enhances plant systemic resistance
4. Materials Science
- Biodegradable Polymers: Modifies thermal/mechanical properties of PLA/PHA blends
- Nanomaterial Synthesis: Stabilizes metal nanoparticles for antimicrobial coatings
5. Industrial Biotechnology
- Fermentation Optimization: Engineered S. cerevisiae strains for high-yield (~25 g/L) production
6. Veterinary Medicine
- Antiparasitic: Treats babesiosis in cattle and aquaculture infections
7. Research Applications
- Radiation Protection: Modulates oxidative stress in preclinical studies
- Analytical Standard: HPLC/GC-MS reference for artemisinin quantification
Artemisinic Acid Powder (CAS No. 80286-58-4) FAQs
Q1. What are its core properties?
The powder melts at 142–145°C and decomposes above 200°C. It dissolves in polar solvents (methanol, ethanol, DMSO) but not in water or hexane. Its structure includes conjugated double bonds sensitive to light/oxidation, causing yellowing upon air exposure. Infrared spectra show carbonyl (1700 cm⁻¹) and carboxylic hydroxyl (3200 cm⁻¹) peaks.
Q2. Is it safe?
Handling requires caution: May cause eye/skin irritation. Ingestion risks include nausea and dizziness. No severe toxicity reported at industrial exposure levels, but avoid inhalation. Not evaluated for pregnancy use. Store away from oxidizers.
Q3. How does it differ from artemisinin?
Artemisinic acid lacks artemisinin’s endoperoxide bridge, making it non-antimalarial but more stable. It serves as the direct chemical precursor; conversion to artemisinin requires photo-oxidation or enzymatic steps.