What Are Surfactants
Surfactants are special chemicals that can make liquids mix more. The word was named as a combination of "surface active agent." These chemicals work by reducing the tension between two unlike materials, like two liquids or a liquid and something solid. Every surfactant molecule contains two prominent parts. One is a hydrophilic group, or water-attracting, and contains groups like -OH or -COOH. The other is a hydrophobic group, or water-repelling but oil-attracting, and contains groups like alkyl chains. These two opposite parts are joined within one molecule.
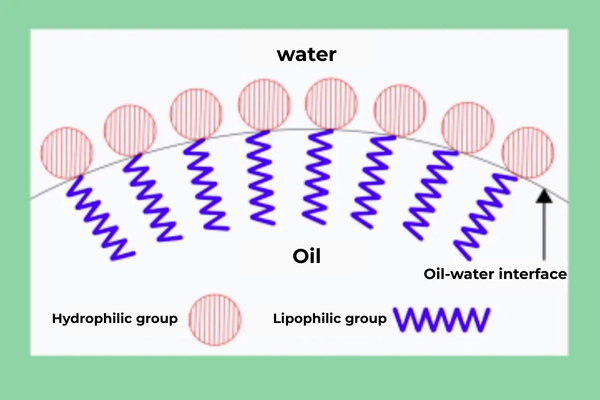
Fig 1. Molecular Structure of Surfactants
This unique shape gives surfactants their special abilities. They get to touch water and oil at the same time, but they don't belong to either one. That's why they're so useful in so many things we put on ourselves daily. The water-attracting side sticks to water, while the oil-attracting side sticks to oils or dirt. Together, these actions help surfactants clean, mix, and do many other important jobs.
Properties and Functions of Surfactants
Surfactants exhibit exceptional efficiency in reducing surface and interfacial tension. Above critical concentrations, they form molecularly ordered assemblies, enabling diverse functional applications.
Properties
- Surface Tension Reduction
Surfactants markedly decrease liquid surface tension. Their molecules align directionally at liquid surfaces, forming monolayers that alter intermolecular interactions and reduce surface tension.
- Micelle Formation
Micelles are aggregates with hydrophobic cores and hydrophilic exterrons, typically adopting spherical, lamellar, or rod-like structures. At low concentrations, surfactants disperse as monomers or adsorb at interfaces to lower tension. When surface saturation prevents further adsorption (Fig. 2a-b), molecules migrate into the bulk solution. Hydrophobic moieties exhibit low affinity for water but strong mutual attraction, leading to self-association into micelles beyond critical concentrations (Fig. 2c-d).
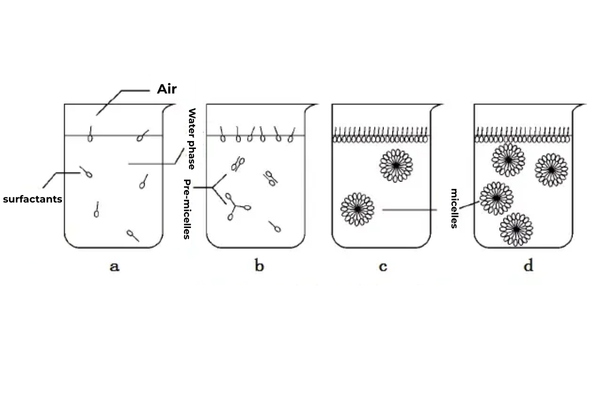
Fig 2. Micellization Process of Surfactants
Functions
These unique properties enable multiple functions:
- Emulsification: Stabilizes oil-water mixtures by surrounding hydrophobic oil droplets with hydrophilic groups, forming homogeneous emulsions and preventing phase separation.
- Wetting: Enhances water spreading on hydrophobic surfaces (e.g., grease, wax) by reducing solid-liquid interfacial tension.
- Solubilization: Hydrophobic substances (e.g., oils) become encapsulated within micelle cores, effectively "dissolving" in water. Solubilization capacity depends on hydrophobic chain length, saturation, and surfactant type.
- Dispersion: Adsorbs onto solid particles to prevent aggregation and stabilize suspensions.
- Foaming: Reduces gas-liquid interfacial tension to promote foam formation and stability.
Types of Surfactants
Since surfactants usually exist in water systems, their hydrophilic groups are dissolved through ionic interactions or hydrogen bonding. So the most common categorization is based on hydrophilic groups. Depending upon the nature of ions formed by the hydrophilic groups, surfactants are classified in four broad categories: anionic, cationic, amphoteric, and nonionic.
Anionic Surfactants
If a surfactant can ionize in water, we refer to it as an ionic surfactant. If the active group on ionization is an anion, i.e., a negatively charged ion, it is called an anionic surfactant. Anionic surfactants are the earliest developed, highest-producing, and most industrialized line of products of this industry. These chemicals have good detergency, but are usually sensitive to hard water.
| Type | General Formula | Representative Varieties | Characteristics |
| Soaps | (RCOO)ₙM | - Sodium stearate - Calcium oleate - Triethanolamine soap | Excellent emulsification and oil dispersion |
| Sulfates | RO-SO₃⁻M | - Sulfated castor oil - Sodium dodecyl sulfate (SDS) - Sodium laureth sulfate (AES) | - SDS: Strong emulsification, acid/calcium tolerance but highly irritating - AES: Hard water resistance, thickening - Sulfated oils: Traditional emulsifiers |
| Sulfonates | R-SO₃⁻M | - Sodium dodecylbenzenesulfonate - Sodium glycocholate - Sodium α-sulfo methyl ester (MES) | - Acid/hydrolysis resistance - High detergency (dodecylbenzenesulfonate) - Biocompatibility (bile salts) |
Cationic Surfactants
Contrary to anionic surfactants, if the active group after ionization is a cation, or a positively charged ion, then it is known as a cationic surfactant. The hydrophilic portion is primarily a nitrogen-containing cationic group, but could be a phosphorus-, sulfur-, or iodine-containing cationic group. A few common compounds are benzalkonium chloride, benzethonium chloride, and benzyl dimethyl ammonium chloride. Cationic surfactants are effective sterilizing, antistatic, softening, and emulsifying agents but poor detergents. Some of their applications are shown in the figure 3 below.
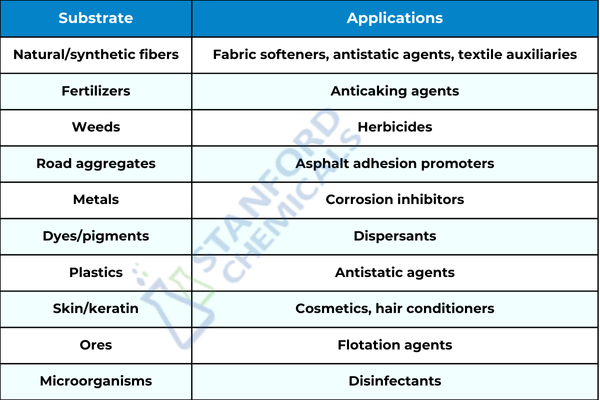
Fig 3. cationic surfactant uses
Amphoteric Surfactant
An amphoteric surfactant is a molecule that ionizes when dissolved in water and possesses a hydrophilic portion with both positive and negative charges at different sites.
Common Varieties:
- Lecithin: Lecithin occurs naturally and is mainly present in soybeans and egg yolks. It is heat-sensitive and hydrolyzed under acidic, alkaline, or esterase conditions. It is insoluble in water but soluble in organic solvents such as chloroform, ether, and petroleum ether. It is one of the key excipients employed in injectable emulsions and lipid particles.
- Amino acids and betaines: They are chemically synthesized. They exhibit surfactant characteristics similar to anionic surfactants in alkaline aqueous solutions with satisfactory foam and detergency. They are similar to cationic surfactants in acid solutions and exhibit excellent sterilization capability.
Amphoteric surfactants cost more to produce, and as such, their market share is comparatively low. Their excellent compatibility and synergy when mixed with others make them extremely flexible in formulation building.
Nonionic Surfactants
The most robust feature of nonionic surfactants compared to the others is that they are unable to ionize in a water solution. Rather, they exist as molecules, not as ions. Their hydrophobic moieties within the molecules are the same as those in ionic surfactants, but their hydrophilic groups are functional groups that can hydrogen bond with water, such as ether groups or free hydroxyl groups. These functional groups occur similarly in general compounds like ethylene oxide, polyols, and ethanolamines.
Advantages:
- Non-ionic surfactants lack acidic groups in their molecular structure, thus preventing precipitation with metal ions and resistance to hard water.
- Their electrically neutral molecules are not affected by strong electrolytes.
- As their molecules do not have acidic or basic groups, their performance remains constant irrespective of solution pH.
- They have very good compatibility with ionic surfactants without any reaction, permitting mixed use.
In the synthesis of nanomaterials, non-ionic surfactants exhibit specific benefits. Their low critical micelle concentration (CMC) makes micelle formation simple in aqueous solutions, resulting in extensive usage in the production of nanoparticles.
Hydrophile-Lipophile Balance (HLB)
The HLB value quantifies the relative affinity of surfactant molecules for water (hydrophilic) and oil (lipophilic). Proposed by Griffin in 1949, it ranges from 0 (paraffin, fully hydrophobic) to 20 (polyoxyethylene, fully hydrophilic). Modern surfactants like sodium lauryl sulfate may reach HLB 40. Higher HLB indicates stronger hydrophilicity; lower values denote greater lipophilicity. Note that molecular structure, temperature, and electrolyte concentration influence practical performance. HLB-Application Correlations:
- W/O emulsifiers: 3–6
- O/W emulsifiers: 8–18
- Solubilizers: 13–18
- Wetting/dispersing agents: 7–9
- Detergents: 13–16
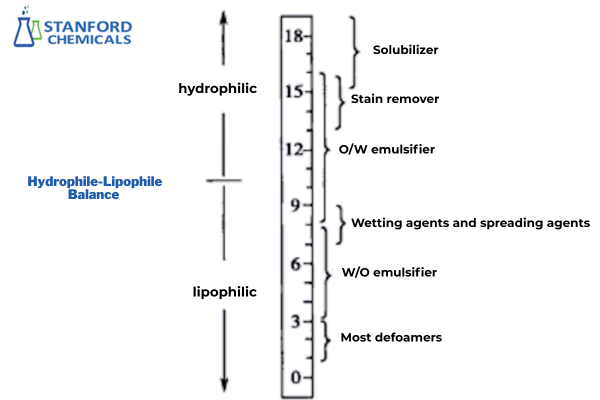
Fig 4. HLB Ranges for Surfactant Applications
Surfactants in Daily Life and Industry
Surfactants are added to many of the products we consume every day. They play an important part in household products like shampoos, soaps, and detergents. Surfactants work to clean by breaking up dirt and grease. Around two-thirds of household surfactant use is applied in personal care products. They are found in hair conditioners, skin creams, and other cosmetics. They are also for even more purposes in factories and businesses. They soften cosmetics and make them easier to put on. Food manufacturers use them to combine ingredients that would not mix otherwise. Drug companies use them to add potency to medication. They're also used to clean hospitals and sterilize equipment. These special chemicals allow many different types of businesses to create better products. From soap in the bathroom to medication in hospitals, surfactants make modern life possible.
For more information on surfactant properties and applications, please contact Stanford Chemicals Company.