In June 2025, a controlled study presented at the International Hyaluronic Acid Conference 2025 in the United States showed that potassium hyaluronate (HA-K) performs better than sodium hyaluronate (HA-Na) in treating dry eye disease. By regulating the ionic balance of the tear film, HA-K can increase tear secretion by 22% and reduce corneal fluorescein staining scores by 35%. Sodium hyaluronate and potassium hyaluronate—what’s the difference between these two similar ingredients?
Sodium Hyaluronate and Potassium Hyaluronate: Derivatives of Hyaluronic Acid
First, A key concept is that both sodium hyaluronate and potassium hyaluronate are derivatives of hyaluronic acid. Hyaluronic acid itself is a large polysaccharide molecule. It has an unstable structure and is difficult to use directly. Turning it into a salt form greatly improves its stability and broadens its applications. So, whether it’s the “sodium” or “potassium” form, the core substance that provides moisturizing and reparative benefits to the skin is still hyaluronic acid. The fundamental difference lies in the cation—sodium ion (Na⁺) or potassium ion (K⁺).
Sodium Hyaluronate and Potassium Hyaluronate: the Difference in Molecular Weight
From a molecular weight perspective, there is no inherent difference between the two. Commercially available sodium and potassium hyaluronate products both cover a full range from low to high molecular weights. Differences in molecular weight do not come from the type of counterion but from the degree of polymerization controlled during manufacturing. Therefore, when discussing molecular weight, the key is to refer to the specific product’s specifications—not to assume one salt type naturally has a higher or lower molecular weight. That said, the counterion does slightly affect hydration capacity, solution viscosity, and ionic strength. For example, at the same concentration and molecular weight, sodium ions have a smaller ionic radius and higher charge density. This allows them to attract water molecules more strongly, forming a thicker and more stable hydration layer. In comparison, potassium ions have lower charge density, resulting in a thinner and looser hydration layer. However, this difference is usually not decisive in practical applications.
| Sodium Hyaluronate | Potassium Hyaluronate | |
| Core Structure | Long-chain hyaluronic acid polysaccharide | |
| Structure | 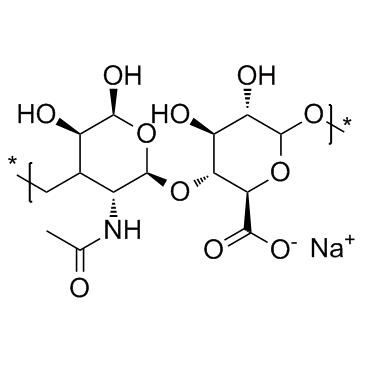 |
 |
| Molecular Formula | C₂₈H₄₄N₂NaO₂₃⁺ | C₂₈H₄₄KN₂O₂₃⁺ |
| Bound ions | Na⁺ | K⁺ |
Sodium Hyaluronate and Potassium Hyaluronate: the Difference in Applications

1. Joint Injections and Medical Aesthetics
In this field, high molecular weight sodium hyaluronate is the dominant choice—especially for joint injections and dermal fillers. Its long-chain molecules form a highly viscoelastic 3D network in tissues. This provides excellent mechanical support and lubrication. In orthopedics, this viscous supplementation therapy effectively relieves joint pain and improves function. In aesthetic medicine, sodium hyaluronate gels are cross-linked to enhance stability and longevity. They are widely used for wrinkle filling, facial contouring, and soft tissue volume restoration. Potassium hyaluronate, on the other hand, is used differently in medicine. Its applications are often linked to the physiological role of potassium ions. A typical use is in certain ophthalmic surgeries, like cataract surgery, where it serves as a component of viscoelastic protective agents. Potassium ions are a key component of aqueous humor and are more compatible with ocular tissues. Potassium hyaluronate is also used in some oral supplements.
2. Skincare
In skincare, molecular weight determines penetration and function. Whether it’s the sodium or potassium form, both follow the same rules regarding molecular weight:
- High molecular weight: Cannot penetrate the skin. It forms a breathable hydrating film on the surface, locks in moisture effectively, and acts as a physical barrier.
- Low molecular weight: Can penetrate into deeper skin layers for intensive hydration.
Potassium hyaluronate is quite common in skincare, especially in formulas that focus on soothing and balancing the skin’s microenvironment. Potassium ions act as co-factors in the biosynthesis of skin ceramides. So, in theory, they can indirectly support skin barrier health.
3. Food and Health Supplements
Oral hyaluronic acid is taken to improve skin hydration and relieve joint discomfort. Studies suggest that low molecular weight hyaluronic acid (including both sodium and potassium salts) may be better absorbed in the intestines. In this area, sodium hyaluronate is the most studied and widely used form, with substantial clinical trial evidence in humans. Potassium hyaluronate is also used in some dietary supplements.
Summary
Both sodium hyaluronate and potassium hyaluronate are derivatives of hyaluronic acid. Their core difference lies not in the polysaccharide structure or molecular weight range, but in the counterion they carry—and the subtle physicochemical and biological effects that result. Sodium hyaluronate holds a dominant position due to its well-established use in medicine and extensive supporting research. Potassium hyaluronate, however, offers unique value in specific cases—such as ophthalmic surgery (where potassium ions play a physiological role) and certain skincare products focused on barrier repair.