When it comes to hyaluronic acid (HA), most people probably think of its use in skincare and joint treatments. SCC has also written many articles on these topics. But there’s more to it. Hyaluronic acid is now recognized as a key material in the development of new biomaterials in the biomedical field. Thanks to its excellent biocompatibility, anti-adhesive properties, and structural versatility, it holds great potential in biomedical applications.
Antibacterial Properties of Hyaluronic Acid
The antibacterial mechanism of hyaluronic acid is the result of both its physicochemical and biological properties. Unlike traditional antibiotics that directly kill bacteria, the unique molecular structure provides HA with a range of indirect yet essential antibacterial functions. 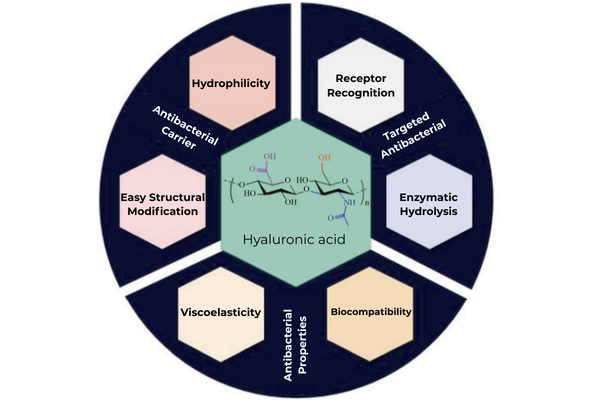
Fig 1. Structure and properties of hyaluronic acid and its application in antibacterial agents
- Anti-Adhesive Effect: This is the most direct and fundamental antibacterial mechanism. Hyaluronic acid molecules enable binding to a large amount of water, forming a highly hydrated, viscoelastic film on the skin or mucosal surface. This film effectively blocks pathogens from contacting epithelial cells, preventing initial bacterial colonization. Since bacterial biofilm formation begins with adhesion, HA stops infection at its source.
- Reduced Bacterial Tissue Permeability: Hyaluronic acid is a major component of the extracellular matrix. However, some pathogens, such as certain streptococci and staphylococci, secrete hyaluronidase, which breaks down HA in tissues. As a result, the extracellular matrix is destroyed and infection is promoted. In response, exogenous HA supplementation can serve as a preventive measure. An excess of hyaluronic acid saturates the hyaluronidase produced by bacteria, preventing it from breaking down the extracellular matrix. This ultimately helps restrict bacterial penetration and spread.
- Immune Regulation and Synergy: High-molecular-weight HA has anti-inflammatory effects. It binds to CD44 receptors on immune cells, triggering cytoskeleton reorganization. This enhances the phagocytic ability of immune cells, helping to prevent excessive inflammation. On the other hand, low-molecular-weight HA acts as a signal released during inflammation, alerting the immune system to respond and clear pathogens.
Applications of Hyaluronic Acid in Antibacterial Formulations
While hyaluronic acid itself is not a potent bactericide, it serves as an excellent antibacterial enhancer and infection preventive agent.
- Targeted Drug Delivery Systems
By virtue of HA's specific binding ability to CD44 receptors, targeted drug delivery systems can be created for infection sites. Evidence shows that the combination of antibiotics like levofloxacin with HA maximizes drug concentration at the infection site significantly, promoting antibacterial activity and reducing systemic toxicity.
- Smart Responsive Formulations
Based on the elevated hyaluronidase activity at infection sites due to bacteria, enzyme-sensitive drug delivery systems can be formulated. These formulations will remain stable in healthy tissue but will break down upon reaching infection sites due to bacterial hyaluronidase activity, delivering the drug specifically. This increases therapeutic response and reduces side effects.
- Wound Dressings and Tissue Engineering
HA-based hydrogel dressings not only possess excellent water retention and gas permeability but also enable the sustained release of antibacterial medicines, creating a microenvironment for wound healing. New materials like silver nanoparticle-HA composite dressings have exhibited remarkable dual properties: antibacterial activity and promotion of tissue regeneration.
Reading more: Why Hyaluronic Acid is an Ideal Material for Wound Healing
- Drug Delivery Carriers
Hyaluronic acid may improve the solubility and stability of many antibacterial drugs and improve their bioavailability by chemical modification or physical encapsulation. It acts as a carrier to reduce drug cytotoxicity and promote more effective therapy for intracellular infections.
Reference: Sodium Hyaluronate Coating for Drug Delivery
Challenges
Although HA shows great potential in antibacterial applications, several challenges remain:
- Endogenous hyaluronidase may prematurely break down exogenous HA.
- Different molecular weights of HA can lead to vastly different biological effects.
- The safety of large-scale clinical applications still requires further validation.
Future research should focus on:
- Developing novel hyaluronic acid derivatives resistant to enzymatic degradation.
- Optimizing the molecular weight distribution of HA-based formulations.
- Exploring synergistic effects between hyaluronic acid and other antibacterial agents.
As a natural biomaterial, HA’s unique antibacterial mechanisms offer broad application value. For more information on the properties and applications of hyaluronic acid, feel free to consult Stanford Chemicals Company (SCC). SCC offers various grades of safe, customizable sodium hyaluronate powder.
People Also Ask
Q: Is hyaluronic acid a disinfectant?
A: No, it's not a disinfectant. It doesn't directly kill germs but prevents infection by forming barriers and supporting the immune response.
Q: Does hyaluronic acid heal?
A: Yes, it heals wounds by suppressing inflammation, keeping the wound moist, and supporting tissue regeneration.
Q: Is hyaluronic acid safe? Can you put it on open wounds?
A: Yes, hyaluronic acid is safe and is used in wound care products to enhance faster wound healing and to create a moist environment.
Q: Is hyaluronic acid antibacterial?
A: Indirectly. It does not kill bacteria but inhibits bacterial adhesion and promotes natural defense mechanisms.