Hyaluronic acid (HA) is a key raw material in modern biomedicine and the cosmetic industry. Its biological functions and application value highly depend on one core parameter—molecular weight. Molecular weight determines the rheological properties, water-retention capacity, permeability, and interactions with cell receptors of HA. Therefore, achieving precise control over its molecular weight is the technological core of producing products that meet different application needs.
Importance of Molecular Weight
The molecular weight range of HA is extremely broad, ranging from several thousand to over two million Daltons. Products with different molecular weights possess distinct physicochemical properties and biological functions:
- High molecular weight hyaluronic acid exhibits strong hydration capacity and can form a highly viscous hydration network. High MW HA offers excellent lubrication, barrier protection, and spatial separation functions. It is mainly used in ophthalmic surgery as a viscoelastic agent, for arthritis injections, and as a film-forming moisturizer in high-end skincare products.
- Medium molecular weight hyaluronic acid combines certain viscoelasticity with tissue permeability. It is commonly used in dermal fillers to increase soft tissue volume and smooth out wrinkles.
- Low molecular weight and oligomeric hyaluronic acid have strong permeability and can be absorbed by the skin or cells. Studies show that specific low MW fragments can influence cell signaling and possess potential bioactivities such as anti-inflammatory, pro-angiogenic, or immunomodulatory effects. Hence, they are used in active skincare products and drug delivery systems.
How to Prepare High‑Molecular‑Weight Hyaluronic Acid
Industrially, high molecular weight HA is predominantly produced on a large scale via microbial fermentation, with the core focus on optimizing the biosynthesis process to maximize polymer chain length.
→ How is Hyaluronic Acid Powder Made
1. Strain Selection and Engineering
High-yield strains such as Streptococcus equi are typically used. Through genetic engineering techniques, the hyaluronic acid synthase gene is overexpressed, while genes encoding endogenous hyaluronidases are knocked out or suppressed, ensuring the synthesis of intact long chains and minimizing degradation from the source.
2. Precise Control of Fermentation Process
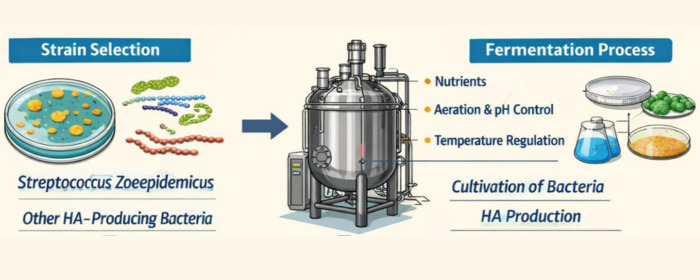
- Nutritional Control: Optimize the culture medium composition, particularly the feeding strategy of carbon sources (e.g., glucose), to ensure an adequate and balanced supply of synthesis precursors (UDP glucuronic acid and N acetylglucosamine).
- Environmental Parameter Control: Precisely maintain temperature, pH, and dissolved oxygen levels in the fermenter. Dissolved oxygen concentration is one of the key factors affecting molecular weight; it is usually maintained within an optimal range to support synthase activity while avoiding chain scission caused by oxidative stress.
- Shear Force Control: Fluid shear forces, due to mechanical agitation, may physically cause long chains to break. Thus, for minimum shear effect, optimization of agitation speed and fermenter design is essential to make sure of mixing and mass transfer efficiency.
3. Gentle Downstream Processing
Following fermentation, rapid inactivation, low temperature operations, and purification steps that avoid strong acid, strong base, or high shear forces are adopted, such as gentle membrane filtration or low temperature ethanol precipitation, to protect the integrity of the polymer chains.
How to Prepare Medium‑Molecular‑Weight Hyaluronic Acid
Two pathways generally exist to obtain the medium MW products: one is a direct fermentation control, and the other is controlled degradation of high MW starting material. 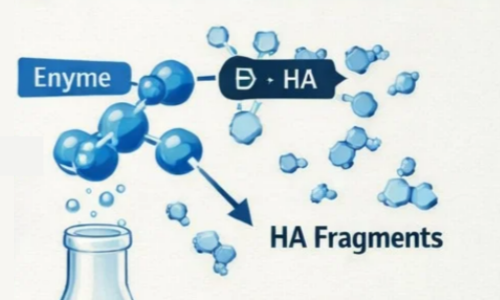
1. Fine‑Tuning via Fermentation Pathway
By manipulation of the above-mentioned fermentation parameters, chain extension can be deliberately restricted. For instance, a moderate rise in fermentation temperature, an altered dissolved oxygen strategy, or the addition of specific nutrient limitations at mid to late fermentation will direct the strain to produce hyaluronic acid within a pre-set molecular weight range. This approach indeed calls for profound knowledge regarding the metabolic network of the strain and process control.
2. Controlled Degradation of High‑MW Starting Material
This is the mainstream approach. Using high MW HA as the starting material, controlled degradation reactions are employed to break it down to the target range. Common methods include:
- Thermal Degradation: Heating an HA solution at a specific temperature (e.g., 80–95°C) for a defined period. The extent of molecular weight reduction is positively correlated with temperature and time. By real-time viscosity monitoring or sampling for molecular weight analysis, heating can be terminated when the target value is reached.
- Mild Chemical Degradation: Treatment with dilute acid or alkali solutions under heating conditions. Relatively controllable degradation can be achieved by strictly controlling the pH, concentration, temperature, and time of the reaction system.
- Physical Degradation: Such as ultrasonic degradation. The mechanical forces generated by ultrasonic cavitation are utilized to cleave glycosidic bonds. Degradation extent is controlled by adjusting ultrasonic power, duration, and solution concentration.
How to Prepare Low‑Molecular‑Weight Hyaluronic Acid
Producing low MW and oligomeric HA requires more intense or more specific chain-breaking methods.
1. Chemical Degradation Methods:
- Oxidative Degradation: Using hydrogen peroxide, ascorbic acid/metal ions (e.g., a redox system composed of vitamin C and copper sulfate), etc. This method is efficient. Adjusting oxidant concentration, temperature, and reaction time, low MW products ranging from several thousand to tens of thousands of Daltons can be obtained in a targeted manner.
- Acid/Alkaline Hydrolysis: Hydrolysis with relatively high concentrations of acid/alkali and higher temperatures. The process is more vigorous, and the molecular weight distribution of the resulting polymer may be quite broad. Careful regulation of the hydrolysis endpoint and prompt neutralization is hence necessary to fix the molecular weight of the polymer.
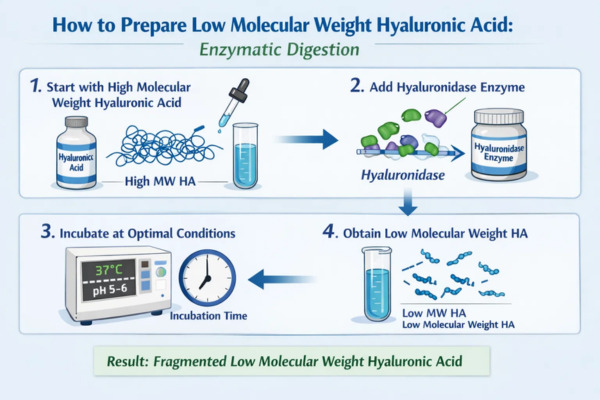
2. Enzymatic Digestion
This is the most accurate method. The method uses hyaluronidase for breakdown. The enzyme hydrolyzes the β1,4-glycosidic bond in HA molecules. The enzyme dose amount, concentration of the substrate, reaction temperature, and reaction time can accurately be controlled so that HA of low MW with narrow MW distribution can be synthesized with defined structures, even in terms of an oligosaccharide of defined degrees of polymerization. The reaction can be accurately terminated by heat inactivating the enzyme when the target molecular weight is achieved.
Risks and Challenges
Although technologies for controlling HA molecular weight are relatively mature, numerous challenges remain in actual production and application.
- Uniformity of Molecular Weight Distribution: Both fermentation and degradation processes yield a mixture of molecules with varying chain lengths. Achieving a narrow molecular weight distribution — a critical requirement for high-end applications such as pharmaceutical injections — demands advanced process monitoring coupled with refined separation and fractionation techniques, including ultrafiltration and chromatography.
- Structure–Activity Relationship: The bioactivity of low‑molecular‑weight HA does not correlate simply with a decrease in molecular weight. Specific molecular‑weight ranges — for instance, hexasaccharide fragments — can exhibit distinct receptor‑binding properties. The targeted production of such specific bioactive fractions remains an active area of research and a persistent challenge for industrial application.
- Precise Control of Degradation Processes: The kinetics of degradation reactions are complex and influenced by multiple variables. Implementing precise control over these processes at an industrial scale typically requires sophisticated analytical instrumentation, such as gel‑permeation chromatography coupled with multi‑angle laser light scattering detection.
- Final Product Safety and Stability: Residual chemical reagents or enzymes from the degradation process must be thoroughly removed to ensure compliance with pharmaceutical and cosmetic safety standards. Furthermore, low‑molecular‑weight HA solutions may demonstrate reduced stability, necessitating stricter controls in packaging and storage.
Summary
The preparation of hyaluronic acid with specific molecular weights is a comprehensive technology that involves microbial fermentation, polymer degradation, process control, and separation/purification. Stanford Chemicals Company (SCC) has the capability to produce HA with a relatively narrow molecular weight range, which allows us to meet customers' customized needs and has demonstrated good performance in practical applications.